How-to calculate the Acid Dew Point (ADP) of flue gas
When flue gas reaches a temperature below the acid dew point, acidic elements will condense. This could cause heat exchanger corrosion. Knowing the acid dew point of your flue gas enables you to select the appropriate materials for your heat recovery installation.
What is the Acid Dew Point?
The acid dew point of flue gas is the temperature at which the acidic elements in your flue gas start to condense.
Why does Acid Dew Point corrosion occur?
The acid dew point of flue gas is the temperature at which the acidic elements in your flue gas start to condense.
Fuel types
Many different types of fuel are used in combustion processes. Used fuels are natural gas, off-gases, LPG, but also naphtha, fuel oils, biogas and solid fuels like coal are applied.
Sulphuric acid components
Most of these fuels contain sulphur components like H2S, mercaptans and thiophenes, which get readily converted to SOx in the combustion chamber. Mainly SO2 is formed, but part of this SO2 (about 2-4%) oxidizes further to SO3.
The rate of this reaction depends on the flue gas temperature; the level of excess oxygen in the combustion chamber, the residence time, the partial pressure of SO2 and the presence of any catalytical surfaces consisting of metal oxides of Iron, Nickel, Vanadium etc.
Sulphuric acid formation below the Acid Dew Point
The formed SO3 subsequently reacts with H2O to form Sulphuric acid, when the flue gas cools below the Acid Dew Point (ADP):
SO3 + H2O -> H2SO4
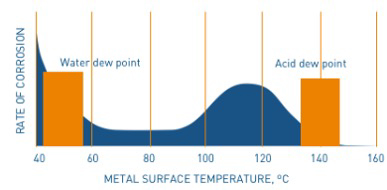
The sulphuric acid is highly corrosive and affects susceptible equipment surfaces. The related rate of corrosion is highest in the range of 100 to 140 ℃. At lower temperatures, the corrosion drops, until water vapor in the flue gas condenses out and forms diluted sulphuric acid:
Acid Dew Point calculation formulas
There are multiple formulas available to accurately calculate the Acid Dew Point. Each formula takes the H2O and SO3 levels of your flue gas into consideration.
Okkes
The Acid Dew Point formula from Okkes:
TDEW (°C) = 203.25 + 27.6log10(pH2O) + 10.83.log10(pSO3) + 1.06.(log10(pSO3) + 8)2.19
Where:
pH2O = volume fraction H2O in m3/m3
pSO3 = volume fraction SO3 in m3/m3
Verhof
The Acid Dew Point formula from Verhof:
TDEW (K) = 1000/[2.276-0.0294.ln(pH2O) – 0.0858.ln(pSO3) + 0.0062.ln(pH2O.pSO3)]
Where:
pH2O = partial pressure H2O in mm Hg (= volume fraction * pressure in mm Hg)
pSO3 = partial pressure SO3 in mm Hg (= volume fraction * pressure in mm Hg)
ZareNezhad
The Acid Dew Point formula from ZareNezhad:
TDEW (°C) = 150 + 8.1328.ln(pH2O) + 11.664.ln(pSO3) – 0.38226.ln(pH2O).ln(pSO3)
Where:
pH2O = partial pressure H2O in mm Hg (= volume fraction * pressure in mm Hg)
pSO3 = partial pressure SO3 in mm Hg (= volume fraction * pressure in mm Hg)
Differences between Acid Dew Point calculation formulas
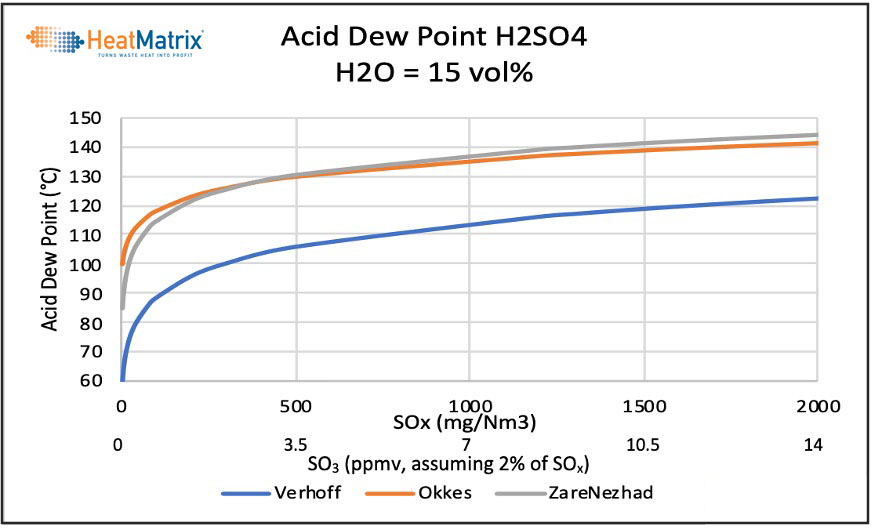
Verhoff’s equation results in a 20 to 40 ℃ lower Acid Dew Point. The ZareNezhad equation closely follows the Okkes equation at higher SO3 levels and calculates a slightly lower ADP at lower SO3 levels.
SO3 itself is hard to analyze for. Typical SO3 levels are assumed to be in the range of 2 – 4% of the SOx level measured. The corresponding graph for the acid dew point related to the SOx level (in mg/Nm3) in the gas, assuming a 2% SO3 content is as follows.
Which Acid Dew Point formula to choose?
Throughout the industry, the ADP correlations of Okkes and ZareNezhad are mainly used. If operation above ADP is desired, it is common to use an additional margin of >10 ℃ above the ADP. This approach provides an engineering basis for a corrosion free operation.
Cooling through the Acid Dew Point without heat exchanger corrosion
Heat recovery potential below the Acid Dew Point
Because of the acid dew point issue, flue gases are normally kept at relatively high temperatures and sent to the atmosphere. That is a pity because the heat from that flue gas can be recovered and reused.
Heat exchanger resistant to acid corrosion
Polymer heat exchangers of HeatMatrix have been designed to be resistant to acid corrosion. This is achieved through the application of Ultra Performance Polymers, specifically chosen for their excellent chemical resistance to corrosive flue – and exhaust gases.
The resistance to acidic solutions has been extensively tested. Chemical resistance tests have been conducted with 80% sulphuric acid at 140 ℃ (sulphuric acid dew point conditions). These tests confirmed the suitability of the polymer for the duty in question.
Keep on learning with these resources
About HeatMatrix
HeatMatrix helps industrial companies map their waste heat recovery options, engineer the most suitable heat recovery systems and implement it in their process. All with one goal: Making the reduction of CO2 emissions, energy consumptions and operational costs as easy as possible.
How can we help you?
We are here to answer any question you might have. We are looking forward to hear from you.